The cleanliness of the liquid copper must be carefully controlled to avoid both the pick-up of impurity elements from the furnace tools and die-casting die, as well as oxygen from the atmosphere.
Most dissolved impurity elements will reduce the electrical conductivity of copper. As shown in Figure 9.3.1, iron is one of the elements that has the largest impact on copper's resistivity, so it is important to prevent the pick-up of any significant concentration of iron or other elements from the furnace tools or the casting die. For example, as little as 0.1% iron dissolved in oxygen-free high conductivity copper will reduce its conductivity from 101% IACS to about 70% IACS, essentially eliminating the benefit of using copper rotors.
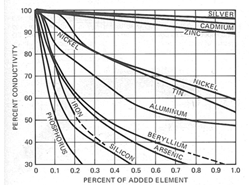 Figure 9.3.1. Influence of dissolved impurity elements on the electrical conductivity of copper at ambient temperature.
Figure 9.3.1. Influence of dissolved impurity elements on the electrical conductivity of copper at ambient temperature. Copyright 1980 Olin Corporation. Used with Permission. All rights reserved
Controlling the oxygen content of the molten copper is also important, as excessive dissolved oxygen can cause cracking of the rotor's conductor bars or end rings. Oxygen is picked up from the atmosphere, and exposing unprotected liquid copper to air for as little as five or ten minutes can cause problems. So unless the melting time is kept short, measures must be taken during both the melting of the liquid copper and subsequent holding at temperature to prevent the pick-up of this detrimental gas. Typically either an inert or slightly reducing cover gas is used over the liquid copper. Nitrogen is normally used as an inert cover, while 5% hydrogen can be added to create a reducing cover.
