 Figure 3A. This lobby in Anchorage, Alaska, artfully displays panels of copper alloys using a variety of colorful chemical and mechanical finishes.
Figure 3A. This lobby in Anchorage, Alaska, artfully displays panels of copper alloys using a variety of colorful chemical and mechanical finishes.Photograph courtesy of CDA.
The wide variety of textures and colors available with copper alloys provide architects with an almost limitless palette of visual effects. In order to systematize this colorful collection and provide a basis for specification, the National Association of Architectural Metal Manufacturers (NAAMM) and the National Ornamental & Miscellaneous Metals Association (NOMMA) describe frequently used finishes in the Metal Finishes Manual for Architectural and Metal Products.1
Field application is occasionally necessary for colored or oxidized finishes and for items such as large immobile statuary. Choice of worksite is normally reserved for the fabricator with consent of the architect.
There are four recognized classes of finishes for copper alloys: mechanical, chemical, protective coatings and laminated finishes. The following paragraphs, abstracted from the NAAMM/NOMMA Manual, describe these and a few other finishes. Use the alpha-numeric codes in Tables 3.7B, 3.7C and 3.7D to specify these finishes.
3.1. Mechanical Finishes
Mechanical finishes are imparted by physical rather than chemical means. As examples, buffing and grinding are mechanical operations; whereas, oxidizing and patinating are chemical in nature.
As-fabricated
These finishes are the mechanical surface conditions resulting from primary production processes, e.g., hot and cold rolling, extrusion, drawing and casting. They are the least expensive finishes, and, while they may contain imperfections, they are uniform enough with sheet goods for applications such as roofing and wall cladding. The term “mill finish” is commonly used for an “as fabricated” finish.
As-fabricated finishes can be marred by secondary operations such as bending, milling and welding, in which case, they may require additional finishing operations. They can also vary in appearance, both intentionally and accidentally. Rolled finishes, for example, will replicate the surface of the final roll in the mill, and as-cast surfaces will betray the nature of the foundry method employed. As-fabricated finishes include:
 Figure 3.1A. As natural a look as you can get: unfinished copper cathode, 99.9% pure. Typically shipped as melting stock to mills or foundries, cathodes may also be cast into wire rod, billets, cakes or ingots and alloyed with other metals.
Figure 3.1A. As natural a look as you can get: unfinished copper cathode, 99.9% pure. Typically shipped as melting stock to mills or foundries, cathodes may also be cast into wire rod, billets, cakes or ingots and alloyed with other metals.Photograph courtesy of CDA.
Unspecified, which place no preconditions on the as-rolled, -extruded, -drawn or -cast metal. For example, unspecified rolled finishes can range from dull to bright and might contain stains imparted by the residues of rolling oils.
Specular, which are bright mirror-like finishes in cold-rolled sheet, strip and plate produced by passing the metal between highly polished steel rolls. Chemical, heat-treated and mechanical design on copper.
Matte, dull finishes produced by hot rolling, extruding, casting or cold rolling followed by annealing.
Polished and Buffed
 Figure 3.1B. The specular or mirror finish on this facade reflects the colors and images of its environment.
Figure 3.1B. The specular or mirror finish on this facade reflects the colors and images of its environment.Photograph courtesy of CDA.
Produced by sequential grinding, polishing and buffing operations, polished and buffed are extremely smooth and bright. Their relatively high cost reflects the added value of preparation. They are frequently used for hardware and small decorative objects. Their high reflectivity imparts a tendency to reveal even slight distortions and lack of flatness. Polished and buffed finishes on interior surfaces are often protected from tarnishing using lacquers. The two subclassifications of buffed finishes are:
Smooth specular, a very bright, mirror-like surface produced by abrasive belt-grinding followed by polishing with progressively finer abrasives and buffing with extremely fine compounds. This is the most costly mechanical finish applied to copper metals. It is especially important to protect smooth specular finishes during installation because they are challenging to apply or touch-up once installed.
Specular is a somewhat less-bright surface. It is also produced by polishing and buffing, but to a lesser extent than that for smooth specular. Surfaces may contain minor scratches and imperfections. Specular finishes are also shop-applied. Field repair is possible but expensive, since it involves extensive hand operations.
Directionally Textured
These finishes are the most frequently used mechanical treatments for architectural copper metals. Their smooth, satin sheen is produced by wheel- or belt-polishing with fine abrasives that leave closely spaced, nearly parallel scratches. The six standard directionally textured finishes are:
Fine, medium and coarse satin, which reflect the coarseness of the final polishing abrasive. Final grits range from 240 mesh to 320 mesh for fine satin and 80 mesh for coarse satin.
 Figure 3.1C. Directionally textured example: C11000, brush finished.
Figure 3.1C. Directionally textured example: C11000, brush finished.Photograph courtesy of CDA.
Uniform, a cost-effective finish produced by a single pass of a No. 80 grit belt. Uniform finishes are less expensive than satin finishes and are suitable for many architectural applications.
Hand-rubbed finishes are produced by rubbing with No. 0 pumice and solvent on a fine brass wire brush or a woven, nonabrasive pad. This is a relatively expensive, labor-intensive process. It is used where other processes are impractical or where there is a need to smooth and blend other satin finishes.
Brushed finishes are produced by power-driven wire- wheel brushes, wire-backed sander heads, abrasive- impregnated fiber pads or abrasive cloth wheels. Scratches produced using brushes are not as uniform as those made with abrasive belts, but brushing offers the advantages that it can be applied to objects with curved or irregular contours. Brushed finishes are difficult to maintain, and their use is normally restricted to small areas or for highlighting.
Nondirectional Textured
These are matte finishes produced by spraying sand or metal shot against the metal surface. Roughness is controlled by size of sand or shot particles. The process is often used to clean castings and improve appearance of an as-cast surface. Sand blasted surfaces are fairly rough, but smoother finishes can be produced by vapor honing with fine abrasive slurries.
 Figure 3.1D. Complementing or enhancing interior spaces, mechanically finished copper alloy surfaces can take on an infinite array of patterns. Their classy look and durability adds value whether in baths, elevators, lobbies or other locations.
Figure 3.1D. Complementing or enhancing interior spaces, mechanically finished copper alloy surfaces can take on an infinite array of patterns. Their classy look and durability adds value whether in baths, elevators, lobbies or other locations.Photograph courtesy of CDA.
Hand-applied sand blasted surfaces have non-uniform textures and are generally not suitable for large areas. Also, the surface deformation caused by blasting leaves residual stresses that can warp thin elements. The process is, therefore, not recommended for flat elements less than ¼-in (6-mm) thick. Even fine-grit blasted surfaces are rough enough to retain oils and dirt, and will show fingerprints. For an untarnished finish, sand blasted surfaces require protective treatment.
The three grades of fineness for sandblasted surfaces are fine matte, medium matte and coarse matte; produced by appropriately sized silica sand or aluminum oxide. Degrees of grit fineness range from 100–200 mesh for fine textures to 20 mesh for coarse.
Shot blasted surfaces are not as rough as sand blasted, but minimum sheet thickness recommendations still apply. Metal shot (usually steel) ranges from S-70 for fine texture to S-550 for coarse. There are three standard grades for shot blasted surfaces: fine, medium and coarse.
Patterned
Textured, patterned and embossed finishes are produced in light-gauge material by passing sheet between two engraved match-design rolls, impressing patterns on both sides of the sheet (embossing) or between a design roll and a smooth one, thus coining only one side of the sheet. Embossing increases the stiffness of the sheet. It also disperses reflections and minimizes marring in service.
3.2. Chemical Finishes
 Figure 3.2A. Chemical treatment opens a wide-ranging palette of colors and possibilities:
Figure 3.2A. Chemical treatment opens a wide-ranging palette of colors and possibilities:
a) artificial finish light bronze on naval brass at the Oklahoma City National Memorial, Oklahoma City, Oklahoma [left image]
b) custom chemical treatment of a naval brass panel [right image]
Photograph courtesy of CDA.
Copper alloy surfaces can be chemically altered to produce a wide range of colored finishes. Application is as much a craft as it is a science, and results can vary with such factors as temperature, humidity, surface preparation and skill. Such variability is not necessarily detrimental; it can, in fact, contribute to a finish’s charm.
Some chemical treatments are simply for cleaning, as in removing process oils or preparation for subsequent operations. Acid-etching can remove oxides formed during annealing or welding, or to produce a matte surface. “Bright-dip” or “pickled” finishes involve immersing the metal in an acidic bath. They are normally used as intermediate steps before final finishing operations.
Conversion Coatings
 Figure 3.2B. The oxidized finish on this cast silicon bronze lockset produces a stable compound that inhibits corrosion and maintains its color.
Figure 3.2B. The oxidized finish on this cast silicon bronze lockset produces a stable compound that inhibits corrosion and maintains its color.Photograph courtesy of Rocky Mountain Hardware.
These comprise the most important class of final chemical finishes. The metal surface is chemically converted to a stable, protective compound, usually an oxide or a sulfide or another compound, to mimic natural weathering. Common conversion coatings include patinas (commonly called "verdegris"), and statuary (oxidized) finishes.2
Patinas are formed by a variety of methods, all accomplishing in minutes what occurs over years in nature. Synthetic patination replicates the initial period of the natural process to the point where a pleasing color develops. Once placed in service, the natural process continues, reinforcing the applied finish. Early field applied synthetic patination methods yielded coatings that were prone to flaking off, nonuniformity in color and staining of adjacent materials. But, the technology has improved considerably, and modern mill-finished products are more uniform and durable. (See some examples in Table 3.5A)
Natural patinas are predominantly mixtures of basic copper carbonate and basic copper sulfate (the latter being the mineral, brochantite). However, compositions are variable and depend strongly on the type and concentration of atmospheric constituents. Thus, patinas formed near the sea contain slightly different percentages of copper sulfates, chlorides and carbonates than those formed in industrial or rural areas. Natural patinas are therefore far from uniform, and architects have learned to take this fact into account.
 Figure 3.2C. Natural patinas are seen throughout the world where architects have chosen to use a living metal that projects beauty in any environment and preserves itself for centuries to come.
Figure 3.2C. Natural patinas are seen throughout the world where architects have chosen to use a living metal that projects beauty in any environment and preserves itself for centuries to come. a) One Atlantic Center, Atlanta, Georgia; copper roof after more than 20 years’ exposure in a humid environment. [left most image]
b) Druid Hills Baptist Church, Atlanta, Georgia, mature copper patina, characteristic of over 80 years’ exposure in a humid location. [vertical center image]
c) Jewish Federation of Tulsa, Oklahoma, newly installed copper. [top right image]
d) Jewish Federation of Tulsa after five years’ exposure in a semi-arid climate. [bottom right image]
Photograph courtesy of CDA.
In addition to atmospheric composition, such factors as temperature, humidity, drainage, insolation and roof slope can affect formation rate and appearance. It would not be unusual for the north- and south-facing surfaces of the same roof to patinate differently or at different rates. A synthetic patina can mitigate these effects; although, one that appears too uniform might look contrived until further, natural weathering takes its course.
Synthetic patinas are normally produced by the action of acid-chloride or acid-sulfate treatments in which the reagents are applied by brushing, sponging, stippling or spraying. Many patinating processes exist, including proprietary, mill-applied versions applied by sheet and strip suppliers. Patented preparations are also available. When large-area uniformity is not critical, two processes found to be suitable for field- or shop-application are:
- Acid chloride treatments are based on solutions of sal ammoniac (ammonium chloride) or on cuprous chloride-hydrochloric acid mixtures. Several applications of the sal ammoniac solution may be needed to achieve the desired effect.
- Ammonium sulfate treatment finishes contain copper sulfate and ammonia. Treatments are applied by spraying and require six to eight applications to achieve the required density.
 Figure 3.2D. Skillful use of chemical treatment techniques provide a great variety of color tones from a given copper alloy.
Figure 3.2D. Skillful use of chemical treatment techniques provide a great variety of color tones from a given copper alloy.Photograph courtesy of Stuart Dean.
Note on chemical weathering in field environments: Chemical coloring of exposed flashings, chimney caps and similar small surface areas are possible with reasonable expectation of success. Chemical weathering of large surface areas, such as roofs, spires, domes and walls, is impractical and should be avoided. Realistic color tone, uniformity and durability is difficult to control, and the hand methods employed are expensive. Where large areas are involved, either natural weathering or use of factory patinated copper sheet material provides superior results.
Patinas are normally reserved for non-traffic areas and areas that will receive little or no maintenance. If placed in a traffic area, a clear organic coating may provide protection, but can alter the color.
Oxide or statuary bronze treatments are based on cuprous oxide, sometimes combined with a mixture of copper sulfides. They are commonly applied to bronzes used as artistic, decorative or, architectural elements. Their brownish colors range from light to medium to dark, depending on the copper alloy, fineness of surface texture and concentration and number of applications of coloring solutions. Popular reagents include potassium permanganate and copper salt solutions.
Finishes are often augmented by mechanical abrasion to produce highlights. Statuary finishes are commonly hand-applied and hand-rubbed periodically with wax or oils to maintain a stable appearance.
 Figure 3.2E. Statuary bronze finishes are popular for many uses, including building entries.
Figure 3.2E. Statuary bronze finishes are popular for many uses, including building entries.Photograph courtesy of Stuart Dean.
Sulfide treatments are similar in color to oxide finishes but are produced by dipping, spraying or brushing the surface with reagents such as potassium sulfide, sodium sulfide, ammonium sulfide and, less frequently, antimony pentasulfide, the latter applied as a paste. Oxide treatments may be applied as a preliminary step to improve adherence.
Selenide treatments produce deep colors. Formation is rapid with use of appropriate selenide solutions, many of which are proprietary. Application can involve exposure to hazardous reagents.
3.3. Protective Coatings
Coatings are finishes applied over copper metals that may or may not have received mechanical or chemical treatment. Coatings are usually applied for protection but, in some cases, may also provide visual effects.
Coatings take two general forms: transparent coatings that preserve natural color, texture and metallic luster of copper metals, and opaque coatings that impart corrosion and abrasion resistance while retaining formability.
Clear Organic Coatings
Copper metals are inherently corrosion-resistant, but thin tarnish films and/or patinas can form over time. Clear organic coatings can retard formation of such films and thus preserve the metals’ natural colors by acting as physical and/or chemi-cal barriers to atmospheric chemicals. These coatings degrade over time, especially in fully exposed exterior applications. When specifying a clear coating, consider effort required to periodically remove the clear coating, refinish the underlying metal and apply a new coating.
 Figure 3.3A. The Anchor Center office building in Scottsdale, Arizona, chose to boast its location in the Copper State by preserving the natural color of freshly milled copper sheet with a clear coating. Due to sun and heat, the finish requires reapplication every few years to maintain its pristine look.
Figure 3.3A. The Anchor Center office building in Scottsdale, Arizona, chose to boast its location in the Copper State by preserving the natural color of freshly milled copper sheet with a clear coating. Due to sun and heat, the finish requires reapplication every few years to maintain its pristine look.Photograph courtesy of CDA.
Clear coatings are compounded from synthetic or natural resins, oils or combinations of the three, usually applied as solutions in a volatile solvent. They can be brush- or dip-applied but are most often sprayed and air-dried (i.e., cured or polymerized), especially for large-area applications. They can also be baked, in which case, a harder, more durable (and a more difficult-to-strip) coating results. Finishing processes are straightforward, but any organic coating will perform best when the underlying surface is properly cleaned and prepared as soon as practical before application.
Inhibited acrylic coatings include those containing protective chemicals in addition to the base resin(s). INCRALAC®-type coatings are the most effective in this class. Based on acrylic lacquers, INCRALAC coatings contain an organic oxygen scavenger, usually benzotriazole or a related compound. The basic composition was developed by the International Copper Research Association, and a number of licensed commercial versions are available. The coatings are normally sprayed and air-dried, although dipping and baking are also approved. Sprayed and air-dried films are normally specified for exterior uses. Baked films are more abrasion resistant and are preferred for interior applications, although periodic maintenance is required.
Prepare surfaces by washing with a cleaning solvent and, for non-specular finishes, abrasive pads. Avoid steel wool (a general precaution for all copper metals) since it sometimes contains a rust inhibitor that can stain copper over time if not thoroughly removed. Alkali cleaning is also effective.
Acrylic coatings without inhibitors provide good abrasion resistance at somewhat lower cost than inhibited versions. They are useful for both exterior and interior applications where wear and exposure to chemical reagents are design considerations.
Alkyd coatings have limited serviceability and tend to yellow outdoors unless modified. They are relatively inexpensive, but must be slow-dried or baked. Exterior performance is improved by compounding with melamine resins. Resistance to chemicals is usually good.
Cellulose acetate butyrate coatings are air-drying, inexpensive and, for interior use, provide fair to good service. They tend to darken when exposed to sunlight.
Epoxy coatings have excellent resistance to impact, abrasion and many chemicals. They are relatively expensive, and application can involve additional costs in that some compositions are two-part compositions requiring compounding on-site, while other versions require heat curing. Interior performance is exceptional, but coatings may darken and chalk when exposed outdoors.
Nitrocellulose coatings are the least expensive, easiest to apply and most common air-drying coatings for mild interior service. Although some of these coatings have limited service life, formulations with alkyd or acrylic resins show improved performance. Exterior use requires stripping and reapplication at approximately yearly intervals depending on exposure conditions. Chemical resistance is low.
Urethane coatings have excellent chemical and abrasion resistance. Cost is moderate to relatively high. The coatings were originally intended for interior use, but modified versions may also be suitable for exterior use. Application entails health risks, and appropriate precautions are absolutely necessary.
Silicone coatings are also relatively expensive, although they provide the best service at elevated temperatures and under severe exposure conditions. Abrasion resistance is moderate, so a topcoat of a more resistant coating may be needed. When exposed to ultraviolet radiation, silicone coatings may discolor unless the composition includes a suitable inhibitor.
Pigmented clear coatings, Pigments are occasionally added to a clear coating to fine tune color match between different alloys.
Oils and Waxes
These coatings can be applied over most chemical finishes to enhance their appearance with richer luster and greater depth of color. The finishes are almost always applied to statuary bronzes, in which case they also protect the underlying oxide/sulfide surface treatment.
 Figure 3.3B. Oil-rubbed bronze gates prepared for installation at the State Capital in Harrisburg, Pennsylvania.
Figure 3.3B. Oil-rubbed bronze gates prepared for installation at the State Capital in Harrisburg, Pennsylvania.Photograph courtesy of Weimann Metalcraft.
The most common oils are lemon oil (U.S.P.), lemon grass oil (Citratus or East Indian), paraffin oils, linseed oil and castor oil. Popular waxes include carnauba wax and beeswax, either of which can be applied as a mixture with wood turpentine. Quality commercial waxes also give good results.
Oil newly installed metals weekly for the first month to build up a sound base. Apply oils and waxes by hand rubbing with a well-saturated cloth, followed by a second rubbing with a clean cloth to remove excess finish. Application frequency depends on the severity of service: every one or two weeks for heavy traffic areas; monthly for moderate and light duty areas.
Vitreous Enamels
These coatings have their place in artwork, decorative objects and some small architectural elements. They are seldom applied to larger architectural works.
Metallic Coatings
 Figure 3.3C. Gilded copper dome crowns the Chapel of St. Basil in Houston, Texas; Niko Contracting, sheet metal installer.
Figure 3.3C. Gilded copper dome crowns the Chapel of St. Basil in Houston, Texas; Niko Contracting, sheet metal installer.Photograph courtesy of CDA.
This approach is occasionally used with copper metals, two common examples being tinned and tin-zinc coatings used mainly on copper for roofing, flashing and exterior wall panels. Another example is gilding, whereby a thin layer of gilt (typically gold) is applied to the copper surface.
Other examples include chromium and nickel electroplating given to copper and brass hardware, fasteners and plumbing goods. Some high-end plumbing fixtures are finished with electroplated gold. Plated layers are normally thin enough to replicate the underlying surface texture.
Heat Treatment
This is usually a custom artistic treatment accomplished by gas-torching the metal surface to create patterns of colors. The gas in the torch combines with the air to cause a chemical reaction with the copper alloy surface.
3.4. Laminated Finishes
Laminated finishes are not common for copper metals, since most are opaque. Clear polyvinyl fluoride (PVF) and polyvinylidene fluoride (PVDF) coatings provide corrosion and abrasion resistance and demonstrate long-time resistance to degradation by sunlight.
3.5. Standard Finish Designations
Classification of metal finishes has evolved over the years, but even early systems are still occasionally used. One of these is the U.S. Finishes Designations System, developed by the U.S. Department of Commerce. It mainly defines finishes for brass and bronze hardware. Although it was officially discontinued decades ago, it is still used by some hardware manufacturers and architects today. Ultimately, the Builders Hardware Manufacturers Association (BHMA) established an industrywide numerical system, which is now widely used for hardware items. In deference to common practice, BHMA cross-referenced its designations to the nearest U.S. Finishes numbers, as shown in Table 3.7A.
 Figure 3.5A. Heat and chemical treatments applied to a bronze statue by experienced metals restoration firm Stuart Dean.
Figure 3.5A. Heat and chemical treatments applied to a bronze statue by experienced metals restoration firm Stuart Dean.Photograph courtesy of Stuart Dean.
In 1967, the Copper Development Association adopted a system of designations widely used by architects that offers simplicity and uniformity. The system recognizes four most common types of finishes: mechanical, chemical, clear organic coatings and laminated coatings, each designated by the letters M, C, O and L, respectively. A specific finish is identified by one of these letters followed by a two-digit number. Tables 3.7B, 3.7C and 3.7D list specific designations for mechanical, chemical and laminated finishes, respectively. Metal finishing being as much a craft as a technology, the “Examples of Method of Finishing” listed in the tables are merely suggestions and are not to be taken as mandatory. Alternate methods are acceptable in all cases.
Specify finishes by their designation code(s), with preparatory and final steps listed sequentially. Thus, M36-C51 defines a uniform, directionally textured mechanical finish treated with a cuprous chloride-hydrochloric acid conversion coating, in this case a synthetic patina. Specifications need not be that detailed, however, and designers can call out only the final finish, leaving the preparatory operations to the discretion of the fabricator or finisher.
The letter “x” appearing in a designation listed in the tables implies that no number, other than the first digit, has yet been assigned to the finish in question. When such a finish is called out, follow the numerical designation with a brief written explanation.
| C11000 Copper | C23000 Red Brass | C26000 Cartridge Brass | |
|---|---|---|---|
| Source: CDA | |||
| Untreated | |||
| Chemical Weathering | |||
| Sulfide "Statuary" Medium (C-55) | |||
| Sulfide "Statuary" Dark (C-55) | |||
| Patinated (C-52) | |||
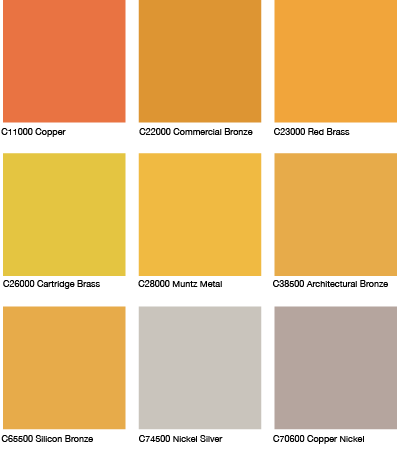
3.6. Copper Alloys Color Chart
A wide variety of copper alloys are available for use in construction. The variations in color stem primarily from differences in chemical composition (see Copper Alloys). Shown below are sheet metal samples of some common copper alloys. Additional information is available from CDA upon request. Note the finishes indicated.
3.7. Finishes Tables
| BHMA Code No. | Description | Nearest US Equivalent |
|---|---|---|
| 605 | Bright brass, clear coated | US3 |
| 606 | Satin brass, clear coated | US4 |
| 611 | Bright bronze, clear coated | US9 |
| 612 | Satin bronze, clear coated | US10 |
| 613 | Dark oxidized satin bronze, oil rubbed | US10B |
| 622 | Flat black coated | US19 |
| 623 | Light oxidized statuary bronze, clear coated | US20 |
| 624 | Dark oxidized statuary bronze, clear coated | US20A |
| 625 | Bright chromium plated over nickel | US26 |
| 626 | Satin chromium plated over nickel | US26D |
| 632 | Bright brass plated, clear coated | US3 |
| Type of Finish | Designation | Description | Examples of Method of Finishing |
|---|---|---|---|
| As-fabricated | M10 | Unspecified | Optional with finisher. |
| M11 | Specular as fabricated | Cold rolling with polished steel rolls. | |
| M12 | Matte finish as fabricated | Cold rolling followed by annealing; also: hot rolling, extruding, casting. | |
| M1x | Other | To be specified | |
| Buffed | M20 | Unspecified | Optional with finisher |
| M21 | Smooth specular | Cutting with aluminum oxide or silicon carbide compounds, starting with relatively coarse grits and finishing with 320 grit using peripheral wheel speed of 6,000 ft/min (30 m/s). Followed by buffing with aluminum oxide buffing compounds with peripheral wheel speed of 7,000 ft/min (36 m/s) | |
| M22 | Specular | Cutting with compounds as for M21 finish, followed by a final light buffing. | |
| M2x | Other | To be specified. | |
| Directionally Textured | M30 | Unspecified | Optional with finisher. |
| M31 | Fine satin | Wheel or belt polishing with aluminum oxide or silicon carbide abrasives of 240–320 grit using a peripheral speed of 6,000 ft/min (30 m/s). | |
| M32 | Medium satin | Wheel or belt polishing with aluminum oxide or silicon carbide abrasives of 180–240 grit using a peripheral speed of 6,000 ft/min (30 m/s). | |
| M33 | Coarse satin | Wheel or belt polishing with aluminum oxide or silicon carbide abrasives of 120–180 grit using a peripheral speed of 6,000 ft/min (30 m/s). | |
| M34 | Hand rubbed | Hand rubbing with stainless steel wool and solvent, #0 pumice and solvent, nonabrasive mesh pad or Turkish oil and emery. | |
| M35 | Brushed | Brushing with rotary stainless steel, brass or nickel silver wire wheel. Coarseness of finish controlled by diameter and speed of wheel and pressure exerted. | |
| M36 | (Number unassigned) | ||
| M3x | (Number unassigned) | To be specified. | |
| Non-directionally Textured | M40 | Unspecified | Optional with finisher. |
| M41 | (Number unassigned) | ||
| M42 | Fine matte | Air blast with #100–#200 mesh silica sand or aluminum oxide. Air pressure 30–90 psi (207–621 kPa). Gun 12 in (305 mm) away from work at an angle of 60–90 degrees. | |
| M43 | Medium matte | Air blast with #40–#80 mesh silica sand or aluminum oxide. Air pressure 30–90 psi (207–621 kPa). Gun 12 in (305 mm) away from work at an angle of 60–90 degrees. | |
| M44 | Coarse matte | Air blast with #20 mesh silica sand or aluminum oxide. Air pressure 30–90 psi (207–621 kPa). Gun 12 in (305 mm) away from work at an angle of 60–90 degrees. | |
| M45 | Fine shot blast | Air blast with S-70 metal shot. | |
| M46 | Medium shot blast | Air blast with S-230 metal shot. | |
| M47 | Coarse shot blast | Air blast with S-550 metal shot. | |
| M4x | Other | To be specified. |
| Type of Finish | Designation | Description | Examples of Method of Finishing |
|---|---|---|---|
| Non-etched Cleaned | C10 | Unspecified | Optional with finisher. |
| C11 | Degreased | Treatment with organic solvent. | |
| C12 | Chemically cleaned | Use of inhibited chemical cleaner. | |
| C1x | Other | To be specified. | |
| Conversion Coatings | C50 | Ammonium chloride (patina) | Saturated solution of commercial sal ammoniac, spray or brush applied. Repeated applications are sometimes required. |
| C51 | Cuprous chloride hydrochloric acid (patina) | In 500 ml of warm water, dissolve 164 g of cuprous chloride crystals, 117ml hydrochloric acid, 69 ml glacial acetic acid, 80 g ammonium chloride, 11 g arsenic trioxide. Dilute to 1 l. Apply by spray, brush or stippling. Repeated applications are sometimes required. Avoid use of aluminum containers. | |
| C52 | Ammonium sulfide (patina) | Dissolve in 1 l of warm water, 111 g ammonium sulfate, 3.5 g copper sulfate, 1.6 g concentrated ammonia. Spray apply. Six to eight applications may be necessary under high humidity conditions. | |
| C53 | Carbonate (patina) | Various formulations having copper carbonate as the major constituent. | |
| C54 | Oxide (statuary) | Principal formulations utilize aqueous solutions of copper sulfates and copper nitrates at temperatures from 85°C to boiling using immersion periods from 30 sec to 5 min. | |
| C55 | Sulfide (statuary) | Apply 2%–5% aqueous solutions of ammonium sulfide, potassium sulfide or sodium sulfide by swabbing or brushing. Repeated application increases depth of color. | |
| C56 | Selenide (statuary) | Proprietary formulations recommended. The solutions are toxic, and user preparation should be avoided. Follow manufacturers’ directions for use without deviation. | |
| C5x | Other | To be specified. |
| Type of Finish | Designation | Description | Examples of Method of Finishing |
|---|---|---|---|
| Film Laminates | Unspecified | L90 | Optional with finisher. |
| Polyvinyl Fluoride | L91 | A one-mil clear film, adhesive bonded to the metal surface. | |
| Other | L9x | To be specified. |
1 NAAMM/NOMMA Metal Finishes Manual, Chapter 2: “Finishes for the Copper Alloys,” National Association of Architectural Metal Manufacturers, AMP 500-06, 2006
2 See also: How to Apply Statuary and Patina Finishes
